In thermodynamics, the Boyle temperature
is the temperature at which a non ideal gas behaves most like an ideal
gas. At Boyle temperature, setting the compressiblity factor 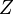 to 1 and one can obtain
to 1 and one can obtain
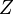 to 1 and one can obtain
to 1 and one can obtain becomes 0. It is at this temperature that the attractive forces and the
repulsive forces acting on the gas particles balance out. Since higher
order virial coefficients are generally much smaller than the second
coefficient, the gas tends to behave as an ideal gas
over a wider range of pressures when the temperature reaches the Boyle
temperature. In any case, when the pressures are low, the second virial coefficient will be the only relevant one because the remaining concern terms of higher order on the pressure. We then have
becomes 0. It is at this temperature that the attractive forces and the
repulsive forces acting on the gas particles balance out. Since higher
order virial coefficients are generally much smaller than the second
coefficient, the gas tends to behave as an ideal gas
over a wider range of pressures when the temperature reaches the Boyle
temperature. In any case, when the pressures are low, the second virial coefficient will be the only relevant one because the remaining concern terms of higher order on the pressure. We then have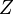 is the compressibility factor
is the compressibility factor


Tidak ada komentar:
Posting Komentar