Oktober 24, 2012
Temperature boyle
3:29:00 PM
| Diposting oleh
Unknown
|
In thermodynamics, the Boyle temperature
is the temperature at which a non ideal gas behaves most like an ideal
gas. At Boyle temperature, setting the compressiblity factor 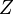 to 1 and one can obtain
to 1 and one can obtain
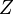 to 1 and one can obtain
to 1 and one can obtain becomes 0. It is at this temperature that the attractive forces and the
repulsive forces acting on the gas particles balance out. Since higher
order virial coefficients are generally much smaller than the second
coefficient, the gas tends to behave as an ideal gas
over a wider range of pressures when the temperature reaches the Boyle
temperature. In any case, when the pressures are low, the second virial coefficient will be the only relevant one because the remaining concern terms of higher order on the pressure. We then have
becomes 0. It is at this temperature that the attractive forces and the
repulsive forces acting on the gas particles balance out. Since higher
order virial coefficients are generally much smaller than the second
coefficient, the gas tends to behave as an ideal gas
over a wider range of pressures when the temperature reaches the Boyle
temperature. In any case, when the pressures are low, the second virial coefficient will be the only relevant one because the remaining concern terms of higher order on the pressure. We then have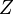 is the compressibility factor
is the compressibility factor
Langganan:
Posting Komentar
(Atom)
.:: Search
.:: Koleksi e-Book
- Physics for scientists and engineers (6ed , Thomson, 2004)
- Fundamentals of Physics
- Fundamentals of physics 9th edition by jearl walker david halliday
- Fundamentals Of Physics 8E (Halliday) Instructors Solution Manual
- Vogels quantitative chemical analysis 5th edition
- Vogel elementary quantitative organic analysis
- Modern analytic chemistry
- Vogels text book of macro and semimicro qualitative inorganic analysis 5th ed
- anorganik_1
- A text book of inorganic chemistry by k newton friend
- Ebook inorganic chemistry pearson miessler tarr 3rd edition
- Students general organic and natural product chemistry
- Wyatt organic synthesis strategy and control
- Writing reaction mechanisms in organic chemistry elsevier
- Vogels text book of practical organic chemsitry
- Vogel arthur a text book of practical organic chemistry
- The art of problem solving in organic chemistry
- Quickstudy organic chemistry reactions
- Quickstudy organic chemistry fundamentals
- Outline of organic chemistry
- Organic chemistry 4th ed paula bruice
- Organic chemistry 2000 oxford clayden
- Organic chemistry morrison boyd
- Organic chemistry by solomon and fhryle 10th ed
- Organic chemistry by john mcmurry
- Kimia organik i jilid 1
- Keynotes in organic chemistry
- Experiments in organic chemistry by fieser 2nd ed
- Dean handbook of organic chemistry 2nd edition
- Basic principles of organic chemistry by john d roberts
- organic chemistry
- guidebook to mechanism in organic chemistry
- atkins_physical_chemistry 8e solutions manual
- biokimia_lehninger
- Vogel elementary quantitative organic analysis
- Bio Kimia Lehninger
.:: Download
- Materi PIL PSBM_SUKUNAN
- Tugas ppt KO karbohidrat
- Makalah PE Glabol Warming
- Makalah PE Biodiesel
- Makalah PE Nuklir
- Makalah PE Bioetanol
- Makalah PE Sel Surya
- Makalah PE Biomassa
- Materi Kuliah KO keynotes
- Materi kuliah KO protein 2
- Materi kuliah KO amina dan amida
- Materi Kuliah KO lipid 3
- Materi KO lipid 2
- Materi Kuliah KO clayden
- Materi Kuliah KO lipid
- Materi Kuliah DKA titrasi kompleksometri
- Materi Kuliah DKA analisa DO
- Materi kuliah KO Karbohidrat
- Materi kuliah titrasi redoks
- Materi kuliah titrasi pengendapan
- Materi kuliah Struktur padatan
- Materi kuliah Amina dan Amida
- makalah PE sel surya
- makalah PE energi
- makalah PE panas bumi
- makalah PE migas
- makalah PE batu bara
- bilangan oksidasi nitrogen
- kekuatan asam dalam medium air
- efek ion bersamaan
- stoikiometri reaksi logam dengan garam
- fotokimia reduksi ion besi(III)
- pemurnian bahan melalui rekristalisasi
- pembuatan kalium nitrat
- efek ion bersamaan
- Laporan praktikum identifikasi gugus fungsi
.:: Followers
Diberdayakan oleh Blogger.




0 komentar:
Posting Komentar